Background: The management of multiple myeloma (MM) continues to evolve with the emergence of new classes of therapy, most recently including BCMA-targeted therapies. Considering the expanding MM treatment armamentarium, we sought to identify contemporary practice patterns among healthcare professionals (HCPs) for MM and compare them with those of experts using an online decision support tool.
Methods: An online decision support tool was developed with input from 5 experts providing therapy recommendations for 192 unique patient case scenarios based on considerations including disease setting, treatment history, access to therapy, and specific comorbidities. HCP tool users entered specific patient criteria to define a case along with their intended management for that case. The tool then showed the 5 expert recommendations for that same case scenario, and users were asked if the recommendations impacted their intended approach. An analysis of expert recommendations and user-selected therapy was performed.
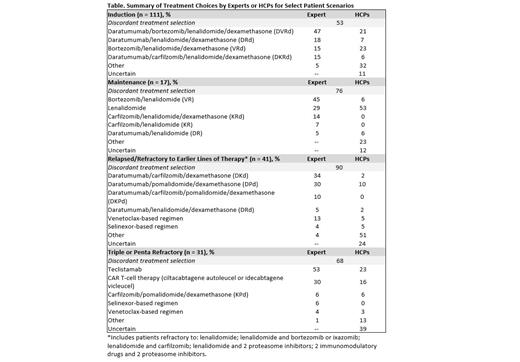
Results: Between April 2023 and July 2023, 207 patient cases were entered by 144 participating HCPs. HCP and expert treatment recommendations were frequently discordant, regardless of treatment setting (Table). The highest concordance between HCP and expert recommendations (47%; P <.0001) was in the induction setting (n = 111). In this setting, experts were more likely to recommend quadruplet regimens compared with HCPs (62% vs 27%, respectively; P <.0001). Treatment selection concordance was lower in the maintenance (n = 17; 24%; P <.0001) and relapsed/refractory settings (n = 79; 20%; P <.0001). The lowest concordance (10%; P <.0001) between HCP and expert treatment recommendations was noted in the relapsed/refractory setting specifically among patients who were refractory to earlier lines of therapy (n = 41; P <.0001). For patients who are refractory to earlier lines of therapy, experts were more likely to recommend an anti-CD38-based combination regimen compared with HCPs (81% vs 32; P <.0001). When recommending treatment for patients with triple- or penta-refractory disease, experts were significantly more likely to recommend BCMA-targeted agents (83% vs 39%; P <.0001).
For HCPs whose treatment plan did not match expert recommendations in these scenarios, 53% of respondents indicated that expert recommendations confirmed or changed their intended therapy, but 31% indicated that there were barriers to implementing those recommendations.
Conclusions: These data suggest ongoing challenges with incorporating the newest therapies and combinations across the spectrum of MM into patient care, particularly multidrug regimens in the induction and maintenance settings and BCMA-targeted therapies in those with triple- or penta-refractory disease. Continued education and development of resources for HCPs, including online decision support tools, may be increasingly important as the treatment of MM continues to grow in complexity.
Disclosures
Lentzsch:Adaptive: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Caelum Biosciences: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Celgene: Research Funding; Clinical Care Options: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Oncopeptide: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Regeneron: Honoraria; Sanofi: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees. Lonial:Bristol-Myers Squibb Company, Janssen Biotech Inc, Novartis, Takeda Pharmaceuticals USA Inc.: Other: Contracted Research, Research Funding; TG Therapeutics Inc: Other: Board of Directors with Stock; Novartis: Research Funding; AbbVie Inc, Amgen Inc, Bristol-Myers Squibb Company, Celgene Corporation, Genentech, a member of the Roche Group, GlaxoSmithKline, Janssen Biotech Inc, Novartis, Pfizer Inc, Takeda Pharmaceuticals USA Inc: Consultancy, Other: Advisory Committee; Janssen: Research Funding. Usmani:Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Moderna: Membership on an entity's Board of Directors or advisory committees; SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; TeneoBio: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; EdoPharma: Membership on an entity's Board of Directors or advisory committees; Array Biopharma: Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Mikhael:Takeda: Consultancy; Sanofi: Consultancy; Janssen: Consultancy; BMS: Consultancy; Amgen: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal